- Organic Chemistry
- Aldehydes and Ketones
- Alkyl Halides, Alcohols and ethers
- Amines and other nitrogen compounds
- Aromatic Chemistry
- Carbohydrates, Amino acids, protein, Vitamin and Fat
- Carboxylic acids and its derivatives
- Chemistry in daily life
- General Mechanism in organic compounds
- Hydrocarbons
- Nomenclature and isomerism
67 - General Chemistry Questions Answers
I want this account closed. I just had to open an account but it’s showing up as a breach to my email. I want an email stating the account has been closed

Joshi sir comment
Constant b is associated to volume correction so its value be less for molecule of smaller volume.
Does stabilization of a charge mean willingness of other atoms in the molecule to share the charge or the extent of sharing of charge ? If its the 1st then how does that stabilize the charge?
Sharing is the case applicable for the atoms of same electronegativity, thus by sharing electrons (charges) atoms make their orbital configurations stable according to the rules defined for stability.


In first case reactions involved areReduction of Fe+++, Fe+++→Fe++Titration step, 6Fe+++K2Cr2O7→6Fe++++2Cr+++By using titration reaction, solve the question
In second case reaction involved isAs4O6+4I3-→As4O10+12I-Now solve
inform if any issue
Balance
KMnO4+H2SO4+H2S -> K2SO4+MnSO4+S+H2O
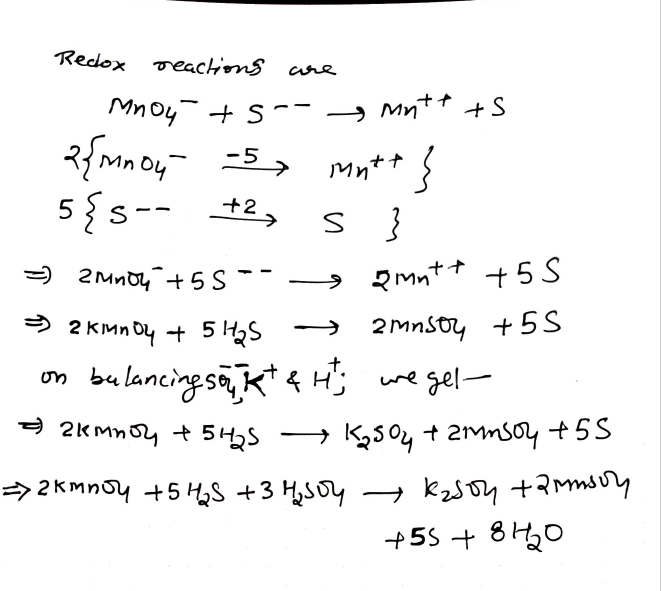
At 752 mm Hg of total Pressure and 301 K Temperature 150 ml H2 is produced and collected over the water, when current is passed through acidic water for 1 hour. How many Amp. current should have passed? ( at 301 K temperature, The pressure of water is 28 mm Hg )
A) 0.320 A
B) 310 A
C) 321 A
D) 0.305 A
please mention correct answer.
by formula PV/T = constant
(752-28)*150/301 = 760*V/273
solve for V, this will be the equivalent volume at STP
now convert this volume in gm by the formula 2V/22400
then finally m = zit, where
z = 1/96500
solve it for i
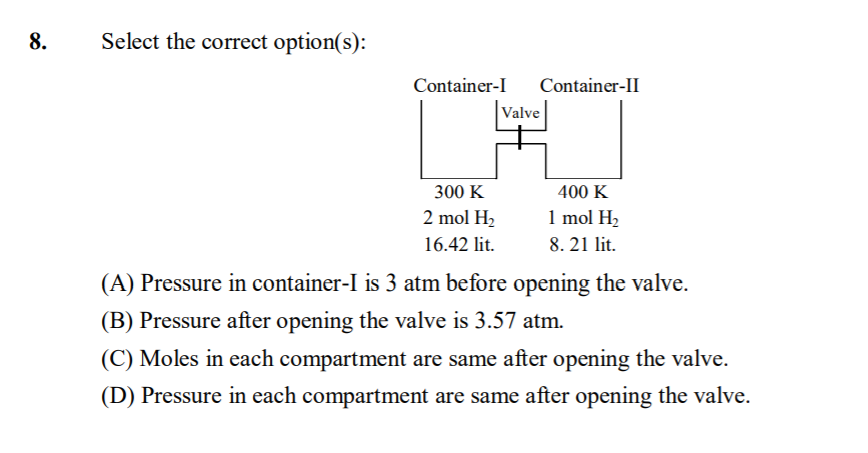
Two flasks of equal volume connected by narrow tube(of negligible volume) are at 300K and contains 0.7 mole of H2(.35 mole in each flask) at 0.5 atm.One of the flask is then immersed in a bath kept at 400k while the other remains at 300K. Caculate the final pressure and the number of moles of H2 in each flask.
by gas eq. PV/nT = constant
so 0.5*2V/0.7*300 = PV/n1400 + PV/n2300 (1)
no. of moles are also constant
so 0.7 = n1 + n2
similarly by gas eq.
PV = n1R400 for first flask
PV = n2R300 for second flask
now solve
Calculate molecular diameter of He from its Van der Waal's constant b=24ml/mole.
b = 4V, where V is volume of 1 mole particles
so 24*10-6 cm3/mole = 4*[4πr3/3] cm3/mole
now solve
The Total energy of one mole of an ideal monoatomic gas at 300K is____.
= 3RT/2