- Organic Chemistry
- Aldehydes and Ketones
- Alkyl Halides, Alcohols and ethers
- Amines and other nitrogen compounds
- Aromatic Chemistry
- Carbohydrates, Amino acids, protein, Vitamin and Fat
- Carboxylic acids and its derivatives
- Chemistry in daily life
- General Mechanism in organic compounds
- Hydrocarbons
- Nomenclature and isomerism
17 - Kinetic Theory of Gases Questions Answers

Joshi sir comment
Constant b is associated to volume correction so its value be less for molecule of smaller volume.
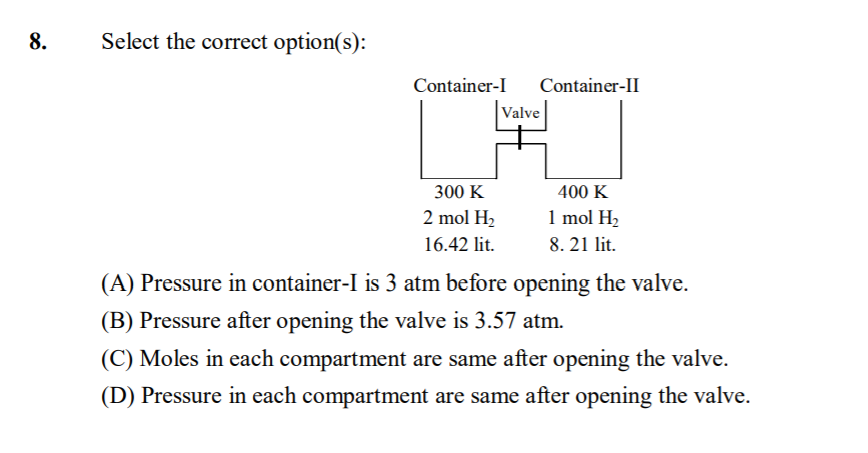
Two flasks of equal volume connected by narrow tube(of negligible volume) are at 300K and contains 0.7 mole of H2(.35 mole in each flask) at 0.5 atm.One of the flask is then immersed in a bath kept at 400k while the other remains at 300K. Caculate the final pressure and the number of moles of H2 in each flask.
by gas eq. PV/nT = constant
so 0.5*2V/0.7*300 = PV/n1400 + PV/n2300 (1)
no. of moles are also constant
so 0.7 = n1 + n2
similarly by gas eq.
PV = n1R400 for first flask
PV = n2R300 for second flask
now solve
Calculate molecular diameter of He from its Van der Waal's constant b=24ml/mole.
b = 4V, where V is volume of 1 mole particles
so 24*10-6 cm3/mole = 4*[4πr3/3] cm3/mole
now solve
The Total energy of one mole of an ideal monoatomic gas at 300K is____.
= 3RT/2
differential form of boyle's law
dV/dP = -V/P
the de- Broglie wavelength of a tennis ball of mass 200g and moving with a speed of 5m/h is of the order
10-20 , 10-30 , 10-40
λ = h/mv
put values and solve but first convert all values in MKS
6.3g of hydrated oxalic acid is treated with M/20 Mg(OH)2. The volume of Mg(OH)2 used for complete neutralisation is
gm eq of oxalic acid = 6.3/63 = 0.1
gm eq of Mg(OH)2 = (1/20)*2*V
now compare the two
In a hydrogen atom electron moves around the circular orbits of radii R and 4R respectively. The ratio of the time taken by them to complete one revolution is
mv2/r = kqe/r2
so v2α 1/r
or (r/t)2α 1/r
or t2 α r3
now solve
There is two atoms of 'P' in one molecule of a compound. If the compound contains 27.93% of phosphorus, the compound will be......
(atomic mass of P = 31)
question is not complete, you should give the information about other elements present.