- Organic Chemistry
- Aldehydes and Ketones
- Alkyl Halides, Alcohols and ethers
- Amines and other nitrogen compounds
- Aromatic Chemistry
- Carbohydrates, Amino acids, protein, Vitamin and Fat
- Carboxylic acids and its derivatives
- Chemistry in daily life
- General Mechanism in organic compounds
- Hydrocarbons
- Nomenclature and isomerism
4 - Redox Reactions and n factor Questions Answers


In first case reactions involved areReduction of Fe+++, Fe+++→Fe++Titration step, 6Fe+++K2Cr2O7→6Fe++++2Cr+++By using titration reaction, solve the question
In second case reaction involved isAs4O6+4I3-→As4O10+12I-Now solve
inform if any issue
Balance
KMnO4+H2SO4+H2S -> K2SO4+MnSO4+S+H2O
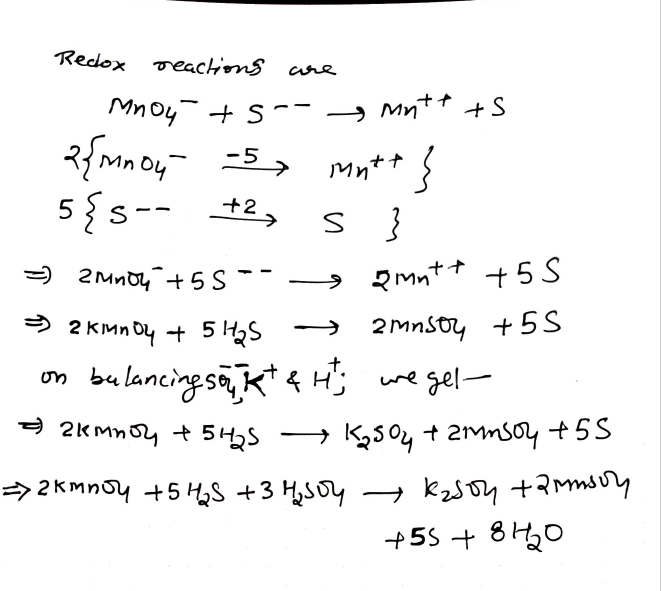
THE E0 VALUES OF FOLLOWING REDUCTn Rxn R GIVEN
Fe 3+ (aq ) + e ----> Fe3+ (aq) E0= 0.771V
Fe2+(aq) + 2e----> Fe (s) E0 =0.447V
WHAT WILL BE THE FREE ENERGY CHANGE FOR D Rxn ?
Fe3+(aq) + 3E----> Fe(s)
ans +11.87kJ/MOL.
Third reaction is the sum of 1st and 2nd so energy of 3rd will be the sum of energies of 1st and 2nd.
delta G = n1FE1 + n2FE2 = 1F(0.771) + 2F(0.447) = F(0.771+0.894) = 1.665F = 1.665*96368 = 160452.7 J = 38203.03 cal = 38.20303 cal
so total energy of the reaction
Fe3+(aq) + 3e- -------> Fe(s) is 38.203 cal
and value of n = 3 so per mole energy = 38.203/3
oxide of E react with KOH & give compd like KMnO4 WHAT IS THE FORMULA OF OXIDE OF E
EO
E2O3
E2O7 OR ANY OTHER ANS (& IF ANY OTHER ONE THEN GIVE THAT ANS WITH SOLn)
- 2 EO2 + 4 KOH + O2 → 2 K2EO4 + 2 H2O
- and
- 3 K2EO4 + 2 CO2 → 2 KEO4 + 2 K2CO3 + EO2
- so oxide will be EO2
